⚠️⚕️ One Lot of Seasonique Birth Control Recalled Due to Possible Extra Yellow Pills (Ethinyl Estradiol Only)
Tuesday, 10 June 2025 08:00.AM
Summary
• Product: Seasonique (DIN 02346176)
• Issue: Health products - Product quality
• What to do: Do not skip doses or stop taking Seasonique. If your package contains an extra row of yellow pills in Tray 1 and/or 2 of the blister cards, do not take them. Instead, return the product to your pharmacy for a replacement or alternative product. If you cannot get to a pharmacy right away, take the next blue-green pill in the proper order as noted in the instructions until you are able to contact your pharmacist and obtain a replacement or alternative product.
Affected product: Seasonique (0.15 mg levonorgestrel, 0.03 mg ethinyl estradiol, and 0.01 mg ethinyl estradiol)
DIN 02346176
Lot 100069151
Expiry 31-Jan-2027
Issue
Teva Canada Ltd. is recalling one lot of Seasonique prescription birth control due to the possibility of having an extra row of yellow pills in tray 1 and/or 2 of the blister cards, where there should be none. This may increase the risk of pregnancy.
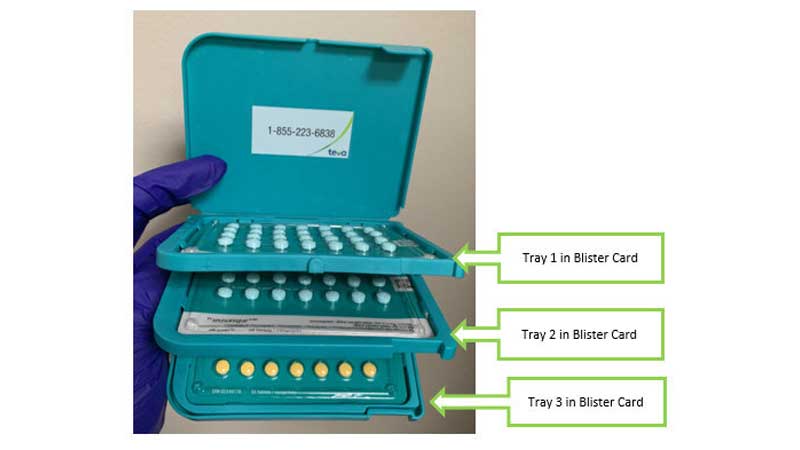
When packaged correctly, as shown in the picture, Seasonique pills come in extended-cycle tablet dispensers, each containing a 13-week (91-day) supply of pills divided in three blister cards:
• Tray 1 and Tray 2 each contain a blister card with 28 blue-green tablets (levonorgestrel and ethinyl estradiol)
• Tray 3 contains a blister card with 35 tablets: 28 blue-green tablets (levonorgestrel and ethinyl estradiol) and 7 yellow tablets (ethinyl estradiol)
Users can expect to have four periods a year, with bleeding occurring while taking the yellow pills.
Seasonique should be taken daily unless otherwise directed by your prescriber. Taking the pills in the proper order, according to the product instructions, is important for preventing pregnancy. Taking the wrong pill or a pill out of order could lead to an unplanned pregnancy or other side effects, including spotting and irregular bleeding.
Health Canada is monitoring the company's recall and investigation, including its implementation of corrective and preventative actions. The Department will inform the public if any new health risks are identified.
Users can expect to have four periods a year, with bleeding occurring while taking the yellow pills.
Seasonique should be taken daily unless otherwise directed by your prescriber. Taking the pills in the proper order, according to the product instructions, is important for preventing pregnancy. Taking the wrong pill or a pill out of order could lead to an unplanned pregnancy or other side effects, including spotting and irregular bleeding.
Health Canada is monitoring the company's recall and investigation, including its implementation of corrective and preventative actions. The Department will inform the public if any new health risks are identified.
What you should do:
• Do not stop taking Seasonique. Do not skip taking any blue–green pills.
• Check your package. If your package contains an extra row of yellow pills in Tray 1 and/or 2 of the blister cards, do not take them. Instead, return it to your pharmacy for a replacement or an alternative product.
• If you cannot get to a pharmacy right away, take the next blue–green pill in the proper order as noted in the instructions until you are able to contact your pharmacist and obtain a replacement or alternative product.
• If you are unsure whether your package contains the correct pills, talk to your pharmacist.
• If you took a yellow pill instead of a blue-green pill from Tray 1 and/or 2, or if you are unsure, you should also use another method of non-hormonal back-up contraception (such as condoms) and consult with your health care professional. Talk to a health care professional if you have any other questions or concerns about your birth control product.
• Contact Teva Canada Ltd. by calling toll-free at 1-800-268-4127, Option 3, or by email at druginfo@tevacanada.com if you have questions about this recall.
• Report any health product-related adverse reactions or complaints to Health Canada
SOURCE: Health Canada
-
Related materials:
- 25-Jan-2026 02:11 PM 🍁💵⚕️ Ontario Opens Homelessness and Addiction Recovery Treatment Hub in Dufferin County
- 03-Jan-2026 12:00 PM ⚕️💊Health Canada Approves TEVIMBRA® (tislelizumab for injection) in Combination with Gemcitabine and Cisplatin for the First-Line Treatment of Adult Patients with Recurrent or Metastatic Nasopharyngeal Carcinoma
- 03-Jan-2026 08:41 AM ⚕️OTO Fertility Launches World's First AI-Powered Platform & Wearable for Predictive Reproductive Insight
- 03-Jan-2026 02:58 AM ⚠️⚕️Public Health Notice: Outbreak Of E. Coli Infections Linked to Pillsbury Brand Pizza Pops
- 19-Dec-2025 12:00 AM ⚕️Health Canada proposes to ban advertising of vaping products wherever they can be seen or heard by youth
- 09-Dec-2025 10:11 AM ⚕️💊 High-risk Early Breast Cancer Patients in Quebec Left Behind by Recent INESSS Recommendation
- 08-Dec-2025 10:27 AM ⚕️💊 With Child Deaths Projected to Rise for the First Time This Century, Gates Foundation Urges Global Leaders to Target Scarce Resources Where They Save the Most Lives
- 07-Dec-2025 04:06 PM ⚠️⚕️Various Pistachios and Pistachio-Containing Products Recalled Due to Salmonella
- 29-Nov-2025 12:00 PM ⚕️💵Canada's $1.5-billion Rare Disease Drug Strategy generating 'return on investment' for patients and society but more needs to be done
- 29-Nov-2025 08:00 AM ⚕️🦷Canadian Dental Care Plan Reaches New Milestone as Government of Canada Strengthens Access to Oral Health Care